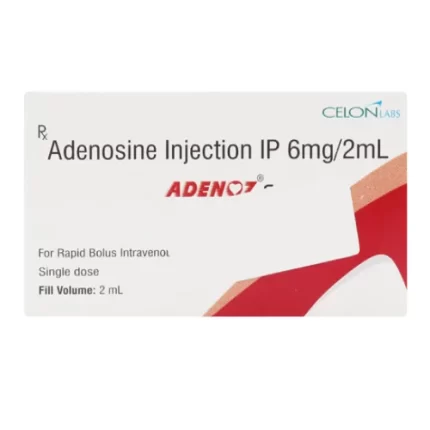
Introduction to Adneon 6mg Injection
Adneon 6mg Injection contains the active ingredient Adenosine. This medication is commonly used to diagnose supraventricular tachycardia (SVT), a rapid heart rhythm disorder originating above the heart’s ventricles. It is administered during electrophysiology studies to induce and assess abnormal heart rhythms, providing valuable information for subsequent treatments. Adneon 6mg Injection is also used in stress tests, such as myocardial perfusion imaging or nuclear stress tests, to evaluate blood flow to the heart muscle.
If an individual has a known allergy or hypersensitivity to Adneon 6mg Injection or its components, its use should be avoided. Inform your healthcare provider about any pre-existing medical conditions, such as gastrointestinal disorders, liver disease, kidney disease, or other health conditions. These may require special precautions or adjustments in the use of this medication. Adneon 6mg Injection is contraindicated in individuals with second or third-degree AV block, as it can further slow down or disrupt the electrical conduction in the heart. This medication can cause transient hypotension (low blood pressure), so it should be used cautiously in individuals with severe hypotension or those at risk of hypotensive episodes.
Uses of Adneon 6mg Injection
- Treatment of arrhythmia
- As a diagnostic aid in the cardiac stress test
Therapeutic Effects of Adneon 6mg Injection
This medication has a therapeutic effect on certain cardiovascular conditions. It is commonly used to diagnose and treat supraventricular tachycardia (SVT). When administered intravenously, Adneon 6mg Injection can temporarily slow down the heart rate and interrupt abnormal electrical pathways, restoring normal sinus rhythm. This can provide rapid relief for individuals experiencing SVT episodes. Additionally, it is used in stress tests to evaluate blood flow to the heart, aiding in diagnosing coronary artery disease. Its ability to dilate coronary arteries helps identify areas of reduced blood flow, indicating potential blockages or narrowing.
Interaction of Adneon 6mg Injection with other drugs
Inform the doctor about your medicines, including prescription, over-the-counter, nutritional or vitamin supplements, and herbal products. Certain medications may interact with Adneon 6mg Injection, reducing effectiveness by causing undesirable side effects.
More Information about Adneon 6mg Injection
- Store at room temperature, between 20°C to 25°C (68°F to 77°F).
- Keep away from moisture, heat, and light.
- It should not be frozen.
- Keep away from children and pets.
How to consume Adneon 6mg Injection
Adneon 6mg Injection is generally administered intravenously (injection into a vein) under the supervision of a healthcare professional. The specific dosage and administration guidelines will be determined based on your individual condition and requirements.
Safety Advices for Adneon 6mg Injection

Pregnancy
The safety of Adneon 6mg Injection during pregnancy has not been extensively studied, and there is limited information available regarding its use in pregnant women. Therefore, healthcare professionals should carefully consider and evaluate its use during pregnancy, weighing the potential benefits against the potential risks.

Breast Feeding
The safety of Adneon 6mg Injection during breastfeeding has not been well studied, and limited information is available regarding its excretion into breast milk. Therefore, caution is recommended when using it while breastfeeding.

Lungs
Adneon 6mg Injection can directly affect the smooth muscle in the airways, potentially causing bronchoconstriction (narrowing of the airways) in some individuals. However, this effect is usually transient and mild. Individuals with pre-existing respiratory conditions such as asthma or chronic obstructive pulmonary disease (COPD) may have a slightly higher risk of bronchoconstriction with the use of this medication.

Liver
Adneon 6mg Injection is generally considered safe for use in individuals with liver impairment. It is primarily metabolized in the liver through the enzyme Adenocar 6mg Injection deaminase. However, it does not significantly affect liver function or cause liver toxicity.

Alcohol
Regarding the safety of Adneon 6mg Injection and alcohol consumption, it is generally recommended to avoid consuming alcohol while taking it. Alcohol can have various effects on the body, including potentially affecting the cardiovascular system and increasing the risk of adverse reactions.

Driving
Adneon 6mg Injection can induce side effects that may compromise your ability to drive safely. These effects, such as dizziness, lightheadedness, blurred vision, and transient changes in heart rhythm, may impact concentration, alertness, and coordination, posing a potential risk while driving.
Side Effects of Adneon 6mg Injection
Adneon 6mg Injection, like all medications, may cause some side effects, although not everyone will experience them.
Serious:
- Severe allergic reactions, including rash, itching, swelling, severe dizziness, or difficulty breathing
- Severe chest pain or angina
- Severe or persistent shortness of breath or difficulty breathing
- Irregular or fast heartbeat
- Fainting or loss of consciousness
- Changes in blood pressure, including severe decrease or increase
- Severe headache or visual disturbances
Common:
- Flushing (temporary redness and warmth of the skin)
- Chest discomfort or chest pain
- Shortness of breath or difficulty breathing
- Dizziness or lightheadedness
- Nausea or stomach discomfort
- Headache
- Sweating
Word of Advice
Advice regarding Adneon 6mg Injection is to always consult with a healthcare professional before starting or making any changes to your medication regimen. They can assess your medical history, evaluate potential risks and benefits, provide personalized guidance, and monitor your progress throughout treatment. Additionally, closely follow the instructions and recommendations your healthcare provider gives, promptly report any unusual or concerning symptoms, and communicate openly about any concerns or questions you may have. It may interact with other medications, such as methylxanthines (e.g., caffeine, theophylline), which can diminish its effects or lead to adverse reactions. Precautions should be taken when using this medication in patients with liver or kidney impairment, as dosage adjustments or special monitoring may be required. The elderly population may be more sensitive to this medication’s effects, and a specialist should determine pediatric use considering the child’s specific condition. Pregnant and breastfeeding individuals should consult with healthcare professionals before using this medication. Your healthcare professional is the best resource for ensuring safe and effective medication use.
FAQs
Q 1. Can I drink caffeine-containing beverages while taking Adneon 6mg Injection?
Limiting or avoiding caffeine intake while using Adenocar 6mg Injection is generally recommended. Caffeine may potentially interfere with the effects of Adneon 6mg Injection, diminishing its effectiveness in diagnosing and treating certain conditions. For specific guidance on caffeine consumption during this medication, it is advisable to consult with your healthcare provider.
Q 2. How long does the effect of Adneon 6mg Injection last?
The effects of Adneon 6mg Injection are generally short-lived, lasting for a few seconds to a couple of minutes. The medication is rapidly metabolized, and its effects wear off quickly. However, the duration of the effect may vary depending on factors such as individual response, dosage, and the specific condition being treated.
Q 3. What are the common side effects of Adneon 6mg Injection?
Common side effects of Adneon 6mg Injection may include flushing, chest discomfort or chest pain, shortness of breath or difficulty breathing, dizziness or lightheadedness, nausea or stomach discomfort, headache, and sweating. These side effects are usually temporary and resolve quickly.
Q 4. Can Adneon 6mg Injection be used during pregnancy for diagnosing or treating heart conditions?
The use of Adneon 6mg Injectionduring pregnancy should be carefully considered, and a healthcare professional should evaluate the potential risks and benefits. The safety in pregnancy has not been well established, and its use should be discussed with a healthcare provider who can provide personalized advice based on the individual’s specific situation.
Q 5. Can Adneon 6mg Injection be used in children?
Adneon 6mg Injection can be used in children, but the dosage and administration should be determined by a pediatric specialist based on the child’s specific condition and needs. The safety and effectiveness of this medication in pediatric patients have been evaluated for certain indications. It’s important to consult with a healthcare professional with pediatric care experience for proper guidance.
Q 6. Can Adneon 6mg Injection be used for heart rhythm disturbances other than supraventricular tachycardia (SVT)?
Yes, Adneon 6mg Injection can diagnose and treat other heart rhythm disturbances besides SVT. It may be used during electrophysiology studies to induce and evaluate different arrhythmias. However, the specific use of this medication for various heart rhythm disorders should be determined by a healthcare professional based on the individual’s condition and needs.
Fact Box of Adneon 6mg Injection
Molecule name: Adenosine
Pharmacological class: Nucleoside Receptor Agonists
Therapeutic class: Antiarrhythmic Agents
Indications:
1. Treatment of arrhythmia
2. As a diagnostic aid in the cardiac stress test
1. Treatment of arrhythmia
2. As a diagnostic aid in the cardiac stress test
References
- Goodman & Gilman’s, The Pharmacological Basis of Therapeutics, Anti-arrhythmic drugs, 12th edition, 2011, 834.
- KD Tripathi, Essentials of Medical Pharmacology, Antiarrhythmic drugs, 7th edition, 2013, 536.
- Wockhardt UK Limited, Electronic medicines compendium (EMC), [ Revised on April 2019] [ Accessed on 10th July 2023], https://www.medicines.org.uk/emc/files/pil.4766.pdf
- Astellas Pharma US, Inc, US Food and Drug Administration, [ Accessed on 10th July 2023], ,https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/019937s026lbl.pdf

 MEDICINES
MEDICINES PATIENT ASSISTANCE PROGRAMS
PATIENT ASSISTANCE PROGRAMS IMPORTED MEDICINES
IMPORTED MEDICINES CONTACT US
CONTACT US Upload
Upload






Reviews
There are no reviews yet.